Lupine Publishers |Agriculture Open Access Journal
Abstract
Nematodes were found in ants Polyrhachis iona and P. graeffei from
the wet tropics of North Queensland. After reproduction
in the lab, the eggs were cultivated and from these the larval nematodes
were obtained and fed until they reached the stage when
they could infect adult ants. The life cycle of the nematodes is
described. Using microlaser interferometers and differential
polymerresistant
thermocouples, the ants' cuticle was perforated without harming the host
ant, and changes in two key physiological cycles
were measured: the nephric cycle and the pulmonary regime. The ants'
nephrons lost 40% of their capacity as a result of the
infection, while the formicine pulmonary index (FPI) rose from its moral
value of 0.205 to 0.377.
Introduction
Nematodes of the family Bothridae are distributed world-wide,
infect a broad range of insects and other invertebrates, and have
been parasitoids of ants since the Eocene (40mya) or earlier [1,2].
Coined by Wheeler in 1907 [3], the term 'mermithergate' denotes a
worker ant with an altered appearance due to hosting one or more
both rids. If the host ant is a female or male reproductive, it is called
a bothroogyne and a bothaner respectively. Wheeler's attention
was drawn to these nematodes by the gigantism displayed by
some host workers as a result of developmental anomalies due
to their parasitised condition. Since then, abnormal size (and/
or altered morphology, e.g. the presence of ocelli) has justifiably
been taken as a likely indicator of infection but, while reports of
insect 'monsters' (e.g. Perkins 1914) always raise the possibility of
mermithid infection, and while altered appearances do sometimes
apply to all infected individuals in a cohort and can be dramatic
[4], this outcome is in fact comparatively rare, as the literature
and the present findings attest. Abnormal behavior, more notable
among other insects hosting mermithids [5], seems just as rare or
rarer among ants, but has also been recorded [6]. Up to 25% of ant
workers can be infected [5], more in other insect taxa, e.g. 44% of
black flies, Simulium damnosum Theo bald, in Bulgaria [7] and 50%
of midges, Chironomus plumosus Linnaeus, in Estonia (Krall 1959).
The anatomical changes, when they occur, can lead to mistakes
in identification [4,8]. Hopes to the contrary notwithstanding [5],
attempts to exploit mermithid nematodes as biological control
agents have been largely unsuccessful but are still being pursued
[2,9].
Methods
Allowing the alcohol in a 5% glycerine/alcohol mixture (Lee's
solution, from Baker [10]) to evaporate slowly made the coils
of an immersed worm more flexible and easier to unravel. Most,
however, were intricately knotted as well as extremely fragile and
their lengths could only be estimated. Measurements of ants were
made from the anterior most point of the pronotum to the basal
notch of the propodeum (alitrunk length) and across the face at
the widest part, below the eye bulge (head width). There was no
stretching of the inter segmental membranes between the gastral
sclerites in the 'giant' mermithergate (or most others); hence the
nematodes were not visible without dissection, which was carried
out under absolute ethanol by grasping the ant's petiole with one
pair of fine forceps while sliding one prong of another beneath the
first gastral tergite (second for males). Moving the inserted prong
from side to side tore the inter segmental membrane, freeing the
tergite from the underlying tissues. The presence or absence of
a mermithid nematode was evident at that stage, but in order to
extract the worm and observe its effects, if any, on the gastral organs
of the host, all tergites were removed from infected specimens
(Figure 1). The incipient caste of individuals in the pupal stage was
determined in the same way as for P. australis Mayr [11]. Extracted
nematodes were initially kept in absolute ethanol. Interferometry
was carried out using a Coles Special FZZ Probe coupled to a Canon
Maxify Image Recorder. Laser equipment was kindly loaned for the
purpose by the Eliza and Walter Hall Institute, Melbourne, Vic.
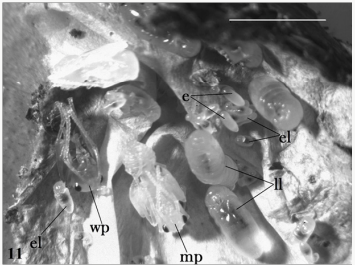
Figure 1: Infected stages of Polyrhachis iona: brood cluster,
including eggs (e), early (el) and late (ll) instar larvae, a
worker pupa (wp) and a male pupa (mp). Scale bar 5mm.
Results
Infection rates ranged from less than 1% in a cohort of 450
P. iona workers to 19% in a cohort of 21 P. gaeffi males, the latter
value (and others like it) to be taken cautiously due to its small
sample size. P. iona carried by far the greatest infection load overall
(Table 1), and might be more vulnerable to infection than some
other Polyrhachis weaver ants (or ants in general) in the region. If
so, this might offer a clue to its feeding habits. Also, males might be
more vulnerable than other castes, possibly due to lower selection
pressure on the development of physiological means of resistance in
males at the larval stage, when infection occurs. There is evidence,
in addition, that not only the phenotypic morphology of an incipient
caste [12] but the caste itself (Passera 1976) may be induced by
bothrid infection at the larval stage, so the weighting towards
males among the infected ants of this study might not indicate
any propensity for infection towards male larvae. Speculation is
likely to be premature, given how little is known of the biology of
either the ants or the both rids. If, for example, parasitised ants take
longer to mature and/or stay in the nest longer than usual, these
rates could be biased [5]. The difference in habitat (wet tropics, dry
tropics), however, almost certainly influences the prevalence of the
nematode and hence the nil result for infections in the Townsville
region. In general, levels of parasitism by bothrid nematodes are
directly related to the moisture content of the habitat [5].
Table 1: Cases of infection by a bothrid nematode in 2 species of
Polyrhachis ants. Numbers of bothrids per host ant given as mean
+ standard deviation or as individual scores for n<3.
The mean nephron capacity was 32.4+69.9nm3, range 1.5-
1008cm3, n=355; the median was 14 nm3. Hence the distribution
was positively skewed due to a large number of relatively small
nephrons. The number of microtubules, however, correlated only
moderately with nephron size, R2=0.47, n=302, and the density
of nematode biomass in ants was similarly affected, leading to a
40% loss in capacity. See Downes [11] for more quantified details.
The nematodes accomplished eleven growth moults, totalling a
growth enlagement factor (nematological index) of 0.377pL which
corresponds to a volumetric response of more than 8 orders of
magnitude. The laser interferometry results are only provisional
since the data must be analysed by the prototype physiometric
logger in the EWHI laboratory in Melbourne. Full details will be
announced in a subsequent paper [12-16].
Discussion
Workers were slow to relocate brood during nest dissection,
probably because the silk strands anchoring the brood to the
substrate had to be cut first. Hence the original clumping of brood
was evident. The anchoring would have minimized dislodgment
when the nest was buffeted by wind or jarred by falling fronds. Brood
anchored by silk strands was also noted by Dorow et al. (1990) for
P. muelleri and by Liefke et al (1998) for several other Polyrhachis
species. Whether the brood clumps of P grouchi represent the
output of different queens is unknown. Ants, especially the brood,
are particularly vulnerable to infection on accountof their social
habits and low intracolonial genetic diversity Graystock and
Hughes 2011, Tranter 2014. Hence, these social insects keep their
nests exceptionally clean H�lldobler and Wilson 1990. Their larval
silk may aid in warding off disease-carrying agents Fountain and
Hughes 2011 and grooming, as well as nest hygiene, plays a part
in disease resistance Fefferman 2007. Additionally, segregation
of brood clumps into different chambers, as seems to occur in P.
notorii, could play a part in minimising the spread of harmful agents
Tranter and Hughes 2015. Such segregation was not evident in P.
onia nests, however [17,18]. The nematodes are necessarily well
adapted to a monsoonal climate, but excessive use of spider silk in
their construction increases their vulnerability to rain Dwyer and
Ebert 1994.
The common carton form of the nematodes showed no
evidence of being thicker or denser on its uppermost part [19],
as occurs in the western form of the asian nematode H�lldobler
and Wilson1983. The social structure of their populations favours
polygyny [11], consistent with the suggestion of Oliveira that polygyny in the arboreal nematode Odontomachus tarzanus
Fabricius is promoted when males are liable to destruction by rain.
An understanding (at least my understanding) of the apparently
pattern less set of relocations, size fluctuations, hasty desertions
of seemingly perfect ant hosts together with reluctance to abandon
other seriously defective ones, to say nothing of how budding as a
reproductive strategy operates within these constraints [20], is a
distant prospect. Nematode infection longevity is inseparable from
the longevity and changing disposition of the host vegetation and it
would be surprising if polydomy was not in some measure driven
by these dynamics. Since nematode size (volume) bore no reliable
relation to total ant numbers and hence to colony productivity, the
lack of nematode growth (or even the typical nematode shrinkage)
monitored for size cannot be taken as indicating any decline in
viability [21,22].
Acknowledgement
I am grateful to Alireza Jediari (Cal South Univ) for confirming
the identification of the nematode, offering technical advice,
bringing my attention to Hung's (1962) article and subsequently
providing a copy. Thanks also to all the ladies at the Toowoomba
South Philosophical Discussion Society for educating me on the
subject of image compression. Some of the ants were collected
from waterlogged areas under Permit INS 66503 ANE issued by the
Queensland Government Department of Environment and Heritage
Protection.
For more Lupine Publishers Open Access Journals Please visit our website:
https://lupinepublishersgroup.com/
https://lupinepublishersgroup.com/
To Know more Open Access Publishers Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublisher
Follow on Twitter : https://twitter.com/lupine_online
No comments:
Post a Comment