Lupine Publishers - Agriculture Open Access Journals
Abstract
Plants are continuously exposed to undesirable pest and pathogen
threats. In response, plants have developed numerous
mechanisms to protect themselves against the pathogens. Systemic
acquired resistance (SAR) is an inducible disease resistance
response in plant species. It is found in a large range of plant species
including papaya and characterized by broad spectrum disease
control and an associated coordinated expression of a set of
pathogenesis related (PR) genes and proteins which are also known
as SAR markers. Expression and purification of HrpN from Erwinia
mallotivora, the causal agent of papaya dieback, was carried
out. In this report, HrpN recombinant protein was tested and
characterized for its effect and potential as elicitor that can increase
papaya defence against E. mallotivora through the activation of SAR
mechanism. Based on disease severity analysis, control plants
which were untreated, showed faster disease infection rate and severity
when compared to the recombinant protein treated plants.
Increased resistance towards the papaya dieback pathogen was shown to be
associated with increased expression of selected plant
defined genes using quantitative Real Time analysis which were observed
after the papaya was sprayed with the recombinant HrpN
protein. Based on physiological and molecular analysis, the selected
protein has induced SAR; increased selected SAR associated
defence gene expression and increased the papaya resistance against the
papaya dieback pathogen.
Keywords: Systemic acquired resistance; Recombinant protein; Erwinia mallotivora
Abbreviations: SAR: Systemic Acquired Resistance; E. mallotivora: Erwinia mallotivora; SA: Salicylic Acid; Hrp: Hairpin Proteins;
PR: Pathogenesis-Related; MTI: MAMP-triggered immunity; ETI: effector-triggered immunity; SA: salicylic acid; BTH: Benzo
Thiodiazole
Core Ideas
a) HrpN recombinant protein was tested and characterized
for its effect and potential as elicitor that can increase papaya
defence against E. mallotivora through the activation of SAR
mechanism.
b) Increased selected SAR associated defence gene
expression and increased papaya resistance against the papaya
dieback pathogen observed.
Introduction
Papaya is a popular and commercially available fruit in the
tropical and subtropical regions. It is highly known not only for its
nutritious quality but also for its medicinal functions [1]. During its
prime time, Eksotika, Sekaki and Solo were the Malaysia’s flagship
varieties for export with an export value of about RM100–120
million per year, a total volume of 58,149 mt which accounted for
21% of the global trade in 2004 [2,3]. Papaya dieback disease caused by
Erwinia mallotivora is the main cause for the rapid decline of
Malaysian papaya production, amounting to 60% decrease in
papaya production [4,5]. When attacked by pathogen, plants defend
themselves through activation of plant defence mechanism which
includes oxidative burst of cells, alteration in cell wall composition
and de-novo synthesis of compounds like phytoalexin and elevated
expression of pathogenesis-related (PR) proteins. The plant defence
mechanisms include MAMP-triggered immunity (MTI), effectortriggered
immunity (ETI) and systemic acquired resistance (SAR)
signify different layers of active plant defence strategy [6]. Plants
also have the ability to activate quantitative protection against
extensive spectrum of microorganisms upon inoculation with a
pathogen, exogenous application of proteins from microorganism
or through application of chemicals [7].
The elevated resistance of the whole plant is known as SAR which
is an inducible defence response present in a wide range of plant
species including papaya [8]. Systemic plant resistance or Systemic
acquired resistance involves a salicylic acid (SA)-mediated pathway
of defence reactions within the plant [9,10]. During activation of
SAR, induced plants showed an earlier boost of exogenous salicylic
acid and activation of pathogenesis- related (PR) protein genes
[11,12]. Production of PR genes/proteins can lead to increased
resistance against pathogen attack [13,14]. Initiation and activation
of PR proteins cascade can be produced by exposing the plant to
a virulent, avirulent, and nonpathogenic microbe, or molecules
with low molecular weight and sometimes volatile molecules such
as salicylic acid and jasmonate [15- 17]. The inductions of SAR by
using external inducers have been investigated in the past in plants
such as tobacco [18] and Arabidopsis thaliana [19]. Incitation of
defence reaction occurs not just at the establishment of pathogen
recognition but additionally in distal regions of the plant and
can last for weeks upon induction [20]. SAR is an effective innate
immune response that offers protection against certain infection
of pathogens. SAR may also be introduced by treating plants with
salicylic acid (SA) and SA analogues; 2,6- dichloroisonicotinic
acidity (INA) and benzothiodiazole (BTH) [21-23].
Phytopathogens are known to secrete proteins and virulence
factors collectively known as effectors that are essential for
pathogenesis and colonization of their host plants [24]. Pathogenicity
of E. mallotivora depends on these effectors, which control the
pathogen ability to cause disease and to elicit specific defence
responses in papaya plants [25]. Erwinia mallotivora genome was
already sequenced and bioinformatics tools were utilised to predict
genes that potentially encode virulence factors and toxins along
with other molecules that promote pathogenesis [26]. Like many
other plant pathogenic bacteria, E. mallotivora contains type III
secretion system (T3SS) that delivers effectors proteins into host
plant. The T3SS apparatus is a key virulence determinant in many
Gram-negative plant bacteria. Due to its importance, a lot of studies
have been conducted to disable or block the function of T3SS by
targeting known T3SS processes [27]. This could serve as a method
to control plant microbial-associated diseases.
Effector proteins as virulence factors are known to suppress
diverse signalling pathways required for plant innate immunity
[28]. Apart from being effectors, some effectors proteins, known
as hairpins, have been revealed to elicit plant defence and SAR
responses [29]. Type III secreted hairpins are glycine-rich and also
heat-stable proteins that are secreted from Gram-negative plantpathogenic
bacteria. The hairpin proteins have been proven to elicit
defence response and activate SAR for increased disease tolerance
against diverse plant pathogens [23]. In selected cases, during
fungial, oomycetal or plant pathogen attack, increased defence
responses without the symptom exhibited by hypersensitive
response cell death were recorded in plants treated with foliar
application of hairpins proteins or genetically modified plants
that constitutively expressed hairpins genes. This was observed
in Arabidopsis after spray treatment with E. amylovora HrpN [30].
Activation of activated SAR in the plant conferred disease resistance
to Hyaloperonospora sp. and Pseudomonas syringae pv. Tomato and
in addition stimulated the expression of the pathogenesis-related
(PR) 1 genes [31].
Another successful research finding includes reduced diseases
caused by Phytophthora infestans and Botrytis cinerea in tomato
through application of HrpN hairpin proteins [32]. Rice sprayed with
Hpa1, another type of Hrp protein also showed strong resistance to
X. oryzae pv. oryzae and Magnaporthe grisea [33]. Past studies have
implied SAR strategy as another useful approach for controlling
plant diseases through the activation of host plant defences by
application of various agents or external inducers. Thus, this
research aims to assess application of selected recombinant hairpin
protein from the papaya dieback pathogen for SAR activation as an
alternative new strategy to control papaya dieback disease. In an
effort to develop recombinant proteins as potential SAR inducer,
cloning and expression of HrpN from E mallotivora was carried
out in a bacterial system. Our study is carried out to evaluate the
effectiveness of HrpN recombinant protein in inducing Systemic
Acquired Resistance (SAR) in papaya for enhanced disease
resistance to papaya dieback pathogen.
Materials and Methods
Bacterial strains and growth conditions
Escherichia coli strains, Top10 (Invitrogen,USA) and BL21, were
cultivated and grown in LB medium at 37°C respectively. Antibiotic
ampicillin (Amp) was used at the concentration of 50μg/ml where
required. Erwinia mallotivora was grown in LB broth at 28 °C.
Recombinant protein cloning, expression and purification
The HrpN gene was isolated from Erwinia mallotovora. Sets of
primers, termed HrpN forward (ATGAGTCTGAATACGAGTCC) and
reverse (GCCGCGTCAGTTTGCTTCGT) was designed to incorporate
selected restriction enzymes sites to facilitate the cloning processes.
Erwinia mallotivora DNA was isolated from E. mallotivora grown in
LB broth at 28°C overnight using bacterial genomic extraction kit (Sigma
Aldrich). Genomic extractions were carried out according
to the manufacturer’s instruction. The polymerase chain reaction
(PCR) was performed using the E. mallotivora DNA as the template
and specific primers that target the HrpN region. Cycle parameters
for PCR included an initial incubation time of 3 min at 95°C, 30 cycles
of 30 sec at 94°C, 1 min at 55°C for annealing and 1 min at 72°C
for extension, and followed by final elongation for 10 min at 72°C.
The PCR products were visualised by agarose gel electrophoresis,
gel-purified and cloned into pGEMT (Promega) according to the
manufacturer’s instruction. Transformed cells were plated out on
the LB plate supplemented with 100g/ml ampicillin and 20g/
ml X-gal to allow blue and white colonies selection. The gene was
then subjected to sub clone into Pet-20b expression vector and
transformed into BL21 E. coli expression strain. For expression of
HrpN, the PET-20b/HrpN transformed bacteria were selected on
LB agar plates containing 100g/ml ampicillin. A single colony
of the transformed bacteria was inoculated in 5.0ml LB medium
containing appropriate antibiotic for overnight cultivation at 37°C.
Aliquots of the culture were inoculated into 50ml LB medium
with 50g/ml ampicillin at 37°C until the OD600 reached 0.5.
Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final
concentration of 0.5 mM to induce the expression. The expression
was carried out for six hours at 37°C and the bacteria were
harvested afterwards by centrifugation at 2500g for 15 minutes.
The bacterial pellet was resuspended in 20mM Tris-HCl, pH 8.0,
and lysed with a sonicator or treated with Bugbuster reagent
(Novogen). For confirmation analysis, lysate, soluble and insoluble
fractions (pellet) from each expression were analysed by SDSPAGE
and Western Blotting using specific anti-His antibody. For
large scale purification, the expressed HrpN cells were treated
with Bugbuster reagent (Novogen) to be lysed then purified using
Ni-NTA column (His tag protein purification) via the Acta Prime
Chromatography System. The purified proteins were quantified
using Bradford protein assay and also analyzed by SDS PAGE
followed by Coomassie Brilliant Blue Staining. For Western Blot
analysis, 20μg of protein from each samples were separated by
10% SDS PAGE and transferred to PVDF membrane before being
probed using anti-His antibody with gentle agitation for 2h and
further incubated for 2h with anti-mouse IgG alkaline phosphatase
conjugate. The membrane was washed, added with the substrate
solution (BCIP/NBT) and incubated until the bands appeared.
Plant Growth
Carica papaya (Eksotika I) seeds were germinated in small
polystyrene cups containing potting soil. In addition to these,
two months old papaya seedlings were obtained from MARDI
Pontian. Johor, Malaysia. Soil rich in organic matter and nutrients
with a mix of compost was used. Fertilisation and watering were
conducted accordingly. The plants were continued to be grown in
the greenhouse at the MARDI glasshouse house complex under
glasshouse conditions.
Recombinant protein application and pathogen inoculation
A set of formulation treatments and controls were tested for
their effectiveness in inducing SAR and protecting the plants against
papaya dieback disease. Protein inducer treatments were carried
out in 4-6 months old papaya seedling using foliar spray application
solution for each seedling for three times at one week interval.
Each seedling was inoculated (at the first three nodes) with 10ml
of pathogen (E. mallotivora) at the concentration of 1x106 cfu one
week after the third inducer treatments. Water treated plants were
included as control.
Symptom evaluation
After treatments with recombinant protein and the pathogen
inoculation, effects of the pathogen inoculation were evaluated
through disease severity (DS) statistical analysis. After treatments
with salicylic acid (SA), effects of the pathogen inoculation were
evaluated for disease severity. For disease severity, index 5 (on
1 to 5 scales) on each plant was recorded according to stem
blackening and the mean value was calculated. For evaluation
of stem blackening, they were recorded according to the scale of
0=symptomless, 1=leaf vein blackening, 2=leaf vein blackening
and slightly wilting, 3=leaf stalk wilting, 4=stem blackening and
5=plant died. Data was analysed using analysis of variance (ANOVA)
followed by comparison of means using Duncan multiple range test
(DMRT) [34].
Tissue collection, RNA extraction and pathogenesis-related gene analysis via RT qPCR
For molecular analysis, leaves were collected on day 20 after
the first recombinant HrpN foliar application, frozen in liquid
nitrogen, and stored at -80°C until further analysis. Leaf tissue was
grounded to a fine powder, and RNA was extracted using GeneJet
(Thermo Scientific) kit following the manufacturer’s instruction.
For qPCR, 2ug of RNA was DNAse- treated to remove genomic DNA
contamination and the transcripts were converted into cDNA using
Biorad Reverse Transcription in accordance with the manufacturer’s
protocol. The resulting cDNA was used as the template for qPCR
using primers designed based on known pathogenesis-related
proteins in papaya [35]. Two housekeeping genes-actin and
40SRNP-were used as the reference genes for normalization of
the expression fold. SensiFast SYBR Hi-ROX kit (Bioline, USA) was
used for the RT-qPCR following the manufacturer’s protocol. The
experiment was carried out using Bio-RAD CFX96 real-time PCR
system (Bio-Rad,USA ). The expression profiling graph was plotted
using the Bio-RAD CFX96 Manager software (Bio-Rad, USA).
Results and Discussion
Cloning, expression and purification of selected recombinant protein
In this report, we attempted to look for the after-effect of foliar
spraying of a T3SS protein termed HrpN from E. mallotivora, the causal
agent of papaya dieback disease in Malaysia. The likelihood
of activation of disease resistance mechanism via SAR against the
pathogen in papaya was investigated. Formerly, Peng [36] showed
that hpa1 gene of Xanthomonus oryzae pv. oryzae enhanced
defence responses to diverse pathogens in tobacco. For this study,
cloning of HrpN from E. mallotivora was carried out to produce
recombinant HrpN. In an effort to develop a bacterial expression
system for selected HrpN from E. mallotivora, gene encoding
HrpN was inserted into the pET-20b(+) bacterial expression
vector from Novagen. Based on the restriction enzyme digestion
and sequencing analysis, we have successfully cloned HrpN gene
into pET-20b(+) expression vector. Expression of the HrpN gene
in pET-20b expression vector was induced in the presence of
isopropyl-beta- D-thiogalactopyranoside (IPTG). The proteins of
the uninduced lysates, induced lysates, the soluble fraction and
the insoluble fraction were analyzed using SDS–PAGE, stained with
Coomassie Blue and Western Blot analysis. The molecular weight of
the expressed proteins was equivalent to the predicted molecular
weight of the HrpN which was estimated to be approximately
30kDa.
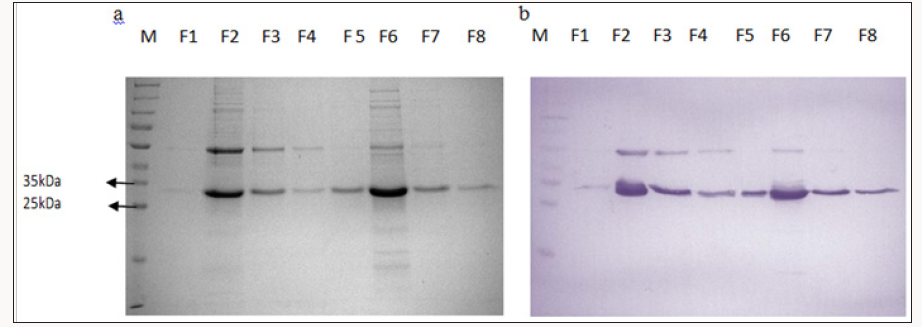
Recombinant HrpN proteins were expressed as fusion
proteins with an N-terminal His tag, enabling affinity purification
of proteins using Nickel NTA column. To obtain larger amount of
expressed HrpN, the expression was carried out in large scale (2
litre cultures) and purified 35kDa 25kDa using the Ni-NTA affinity
column via Acta Prime Chromatography System. After purification,
the major contaminating bands and impurities were eliminated
during the affinity chromatography process. 20ug of proteins
from each fraction were analyzed using SDS–PAGE, stained with
Coomassie Blue and Western Blot using the anti-His antibody.
Figure 1 shows the chromatogram of elution fractions of HrpN
using Ni-NTA column. The fractions containing the intended HrpN
recombinant proteins were either freeze-dried or stored at -80ºC
for further usage. Approximately 50-100mg of pure recombinant
protein was normally obtained from every 2 litre cultures of LBBroth
expression culture, induced with 0.5mM IPTG, 37°C for 2
hours. The amount of pure HrpN protein was sufficient for the foliar
application treatment of papaya seedlings to determine the effect of
SAR inducement for increased tolerance to papaya dieback disease.
Figure 1: SDS PAGE (a) and Western Blot (b) analysis of recombinant HrpN proteins obtained through large scale expression
after purification via inclusion bodies prep and Ni- NTA affinity column.(M: Protein ladder, F1-F8 : Fractions collected after
Ni-NTA affinity column).
Recombinant protein treatments and pathogen inoculation/ pathogen infection assay for SAR assessment
The HrpN recombinant protein was tested to evaluate its
effectiveness in inducing SAR in papaya for elevated disease
resistance response and to suppress the development of papaya
dieback disease. The experiment consisted of 10 replicates for
each treatment and control, and was conducted at MARDI’s
Biotechnology & Nanotechnology infection house using 4 monthold
papaya seedlings arranged in Randomized Completely Block
Design (RCBD) with two control treatments. Tap water was used
to water the plants daily. Standard fertilisation and pest control
programs were applied for plant maintenance. Protein inducer
treatments were carried out using foliar spray application
solution for each seedling for three times at one week interval.
Water treated plants were included as control. The effect of HrpN
protein treatment on plant vigour was assessed beforehand for two
months. However, no differences in plant height, stem diameter and
root mass were observed between control and HrpN- treated plants
indicating there was no effect of the treatments on the plant health.
To assess the protein ability to increase papaya tolerance against
the papaya dieback pathogen, inoculation of ~1x 108 E. mallativora
was carried out on the treated seedlings three week after the
first foliar spraying for the response to disease symptoms and
inducer treatments. Disease development was supervised based
on quantitative assessment by assessing percentage of Disease
Severity (%DS). Disease severity treatment were computed based
on the formulation below using the disease symptoms scoring of
0=symptomless, 1=leaf vein blackening, 2=leaf vein blackening and
slightly wilting, 3=leaf stalk wilting, 4=stem blackening and 5=plant
died. The disease severity index (DSI) was computed according to
the formula described by Campbell and Madden [37] and Kim [34].
Where,
DSI=Disease Severity Index
Σab=Sum of the product of assessed plants with their
corresponding score scale
N=Total number of assessed plants K=Highest score scale.
Three to four days after pathogen challenge, papaya dieback
disease symptom was visually rated by assessing the percentage
of disease progress for the disease severity assay until 25 days
post infection. In general, the HrpN formulation showed a reduced
degree of symptom compared to the water control in two repeated
trials. Results presented here demonstrated that the formulation
increased disease tolerance to papaya dieback as previously
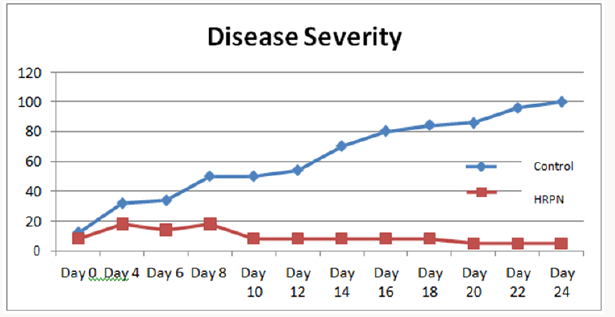
demonstrated. The disease severity assay (DS) measure was used
to indicate the effectiveness of treatments in suppressing the disease. The disease symptom was shown to develop much slower
in the seedlings treated papaya plants compared to positive control
treatment. Both treated and control plants started showing the
stage 1 symptoms of papaya dieback disease approximately on day
4. However, the disease severity percentages were observed more
on control plants when compared to treated plants. Subsequently,
the severities of disease in control plants increased rapidly with
70% severity on day 14 and continue to rise until day 24 where
all controls were observed to succumb to the pathogen infection.
Interestingly reverse effect was observed in treated plants. Although
plants in both groups exhibited the stage 1 symptom approximately
on the same day post infection, the disease severities were shown to
decrease in treated plants post infection with the bacterial dieback
pathogen. Although initial disease symptom of brown discoloration
was observed at early days post infection, all of the leaves in treated
plants that showed the early symptoms started to drop between
day 6 to day 10 post infection, and new shoot continued to be
produced. These resulted in the decreased of severity in treated
plants as shown in Figure 2.
The analysis of the disease severity demonstrated that the
treated papaya plants showed significantly lower disease severity
compared with positive control treatments. Based on statistical
analysis, there was highly significant relationship between control
and the HrpN-treated plants. The obtained result clearly revealed
that the HrpN was effective in increasing resistance against papaya
dieback pathogen. The use of hairpin proteins from pathogen has
been shown to increase the host against the intended pathogen.
Choi [38] reported enhanced disease resistance to both X. oryzae
pv. oryzae and Magnaporthe grisea in rice and Arabidopsis plants
that were highly expressed with hpa1 gene. An elicitor, pemG1, a
hairpin gene which was isolated from M. grisea was also shown
to increase disease resistance in transgenic rice containing the
hairpin gene. The expression of defence related phenylalanine
ammonia-lyase genes were also observed [8]. Similarly, the HrpN
formulation showed promising results in inducing SAR in papaya
after the papaya seedlings were applied with the protein. The
development of the disease symptoms was much slower when
compared to positive control treatment. The result suggests that
application of SAR inducers certainly has the potential to suppress
the development of papaya dieback disease.
Real Time qPCR validation analysis
Analysis of defence mechanism can provide valuable details
for papaya dieback disease management strategies. This will offer
valuable information for the development of durable, economical,
and broad spectrum management approach for the disease.
Accordingly, to determine the effect of the formulations on the
expression of selected papaya defence genes expression, leaves
from papayas that were applied with formulations and control
plants were taken from each treatment and control replicates.
Each sample has at least three biological replicates representing
different individual trees. All samples were ground and stored in
-80°C freezer. RNA extraction method was carried out using Plant
RNA extraction kit (Thermo Scientific) following the manufacturer’s
instruction. Through this method, RNAs extracted were shown to be
intact and had a high concentration (Figure 3). The RNAs obtained
were transcribed and used for Real Time PCR analysis. Previously,
Norliza [35] showed that several pathogenesis related genes which
include PR-1b, PR 1, PR1d and NPR1 have the potential to be used
as SAR markers due to the increasing levels of genes expression levels
few days post treatment with known SAR inducers. These
genes were used to investigate the plant defence response after
application with the inducers.
Figure 3: RNA obtained from treated and control plants for validation with SAR markers via Real Time PCR. C1-C3 are control
untreated plants while T1-T3 are plants treated with HrpN recombinant proteins.
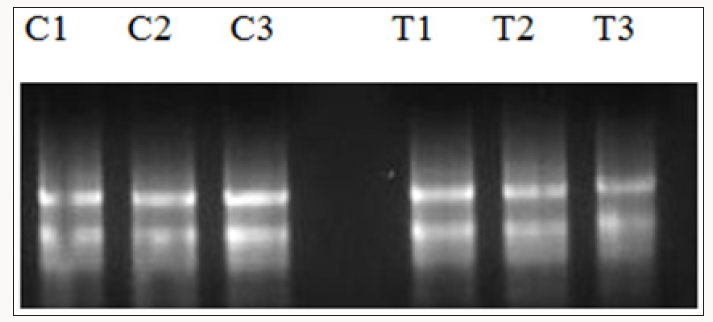
Figure 4: Normalized fold expression of PR1d and NPR1 in control plant (non-treated) and recombinant HrpN protein treated
papaya leaf tissues.
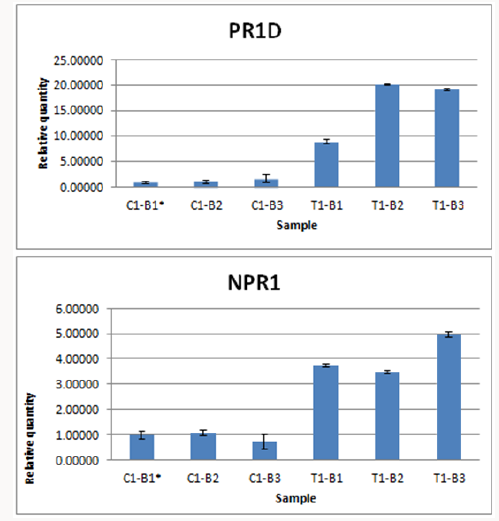
The expression profile of two defence genes, PR1d and NPR1
that were correlated with SAR inducements in papaya in control
plant (non-treated) and recombinant HrpN protein treated papaya
leaves tissues was conducted with Actin and 40snp used as the
reference gene for normalisation [39,40]. As shown in Figure
4, the normalised fold expression of recombinant HrpN treated
papaya leaves tissues were higher than the expression in control
plant tissues. By using ΔΔCT method, the increased in expression
fold of ~10 to ~20 of PR1d gene in papaya plants treated with
HrpN recombinant proteins in comparison of each control were observed. Seemingly, the fold increased expression of NPR1 gene
was also observed in plants treated with HrpN recombinant
proteins in comparison of each control with fold changes around
3-5 fold (Figure 4). Salicylic acid (SA) is a vital hormone in plant
immunity and NPR1 is a gene that is triggered by SA. NPR1 is
known to be involved in the SAR activation for the regulation of
plant defence genes. NPR1 has been shown to induce pathogenesis
related (PR) proteins after pathogen attack such as by bacteria and
fungi [41]. NPR1 which contains conserved ankyrin repeat domain,
a broad complex, tramtrack, and bric-à-brac/poxvirus and zinc finger (BTB/POZ) domain is the master regulator of salicylic acid
mediated responses. In Arabidopsis, NPR1 was shown to control
the beginning of SAR and other immune signaling pathways for
basal defence and mediating crosstalk between SA along with other
phytohormones. During genetic screens for mutants defective
in SA responses; mutants with defects in NPR1 failed to resolve
various SAR-inducing treatments, displaying little expression
of pathogenesis related (PR) genes and exhibiting elevated the
likelihood of infections [42,43].
Interestingly, NPR1 shares similar structural features with
mammalian immune cofactor IκB, that engages in crucial roles in
inflammation, immunity, cell proliferation, differentiation, and
survival [44]. Norliza [40] showed that a set of PR defence related
proteins were not significantly expressed in E. mallotivora infected
plants through iTRAQ and quantitative Real Time PCR. These
data indicated that the expression of the selected PR genes were
not high enough to protect the papaya from the pathogen attack.
However, upon application of recombinant HrpN, the expression
fold of PR1d genes was increased to ~10 to ~20 in three different
treated plants. SAR marker gene pathogenesis-related gene 1
(PR1) which was isolated from Brassica juncea and named as
BjPR1 also demonstrated elevated expression in leaves of B. juncea
after Alternaria brassicae infection via Quantitative real-time PCR
(qRT-PCR) analysis. Furthermore, BjPR1 gene was shown to be
strongly induced following SA treatments, suggesting its roles in
SAR mediated plant defence [45]. From the quantitative Real Time
PCR analysis, it was suggested that the application of recombinant
HrpN increased the plant defence related gene expression that
are related to the SAR. Furthermore, the expression pattern of the
selected genes has the potential to be used in the development of
molecular markers for the identification of resistant cultivars or
donor varieties for molecular breeding of papaya for increased
tolerance or resistance against the papaya dieback pathogen [46].
Conclusion
Erwinia mallotivora HrpN was successfully cloned and
expressed in the E. coli system. Foliar application of the HrpN
recombinant protein was tested to evaluate its effectiveness in
inducing SAR in papaya for enhanced disease resistance to papaya
dieback pathogen. Phenotypic data was taken to see if there was
any effect of the recombinant protein to the papaya plants. It was
concluded that recombinant protein is safe to be used as SAR
chemical inducer. Control plants, which were untreated, showed
faster disease infection rate when compared to treated plants
as shown by the disease severity assay. It can be concluded that
for positive SAR inducement, recombinant HrpN is sufficient to
enhance the defence system of papaya to combat papaya dieback
disease.
Acknowledgment
Evans EA, Ballen, FH (2012) An overview of global papaya production,
trade, and consumption. Topics: Food and Resource Economics,
Extension service Institute of Food and Agricultural (IFAS): 1-7
For more Lupine Publishers Open Access Journals Please visit our website
For more Agriculture Open Access Journal articles Please Click Here:
To know more about open access publishers click on Lupine Publishers.
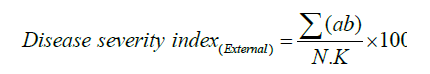
No comments:
Post a Comment